We have a passion for unconventional solutions that bring your vision to life.
A cleanroom is a controlled environment designed to keep airborne dust and microorganisms within strict limits. This is achieved through advanced filtration systems, positive air pressure, air purifiers, and rigorous operational protocols. Cleanrooms are vital in industries like pharmaceuticals, electronics, and biotechnology, where they help maintain product quality, improve production efficiency, and meet high hygiene and safety standards. The International Organization for Standardization (ISO) provides a classification system to evaluate and categorize cleanroom cleanliness levels. This system helps companies maintain consistent production environments and meet required cleanliness standards. This article explores the ISO 8 cleanroom classification in detail.
The ISO 8 classification falls under the ISO 14644-1 standard, which outlines air cleanliness requirements for cleanrooms and controlled environments. This international standard measures cleanliness based on the concentration of airborne particles.
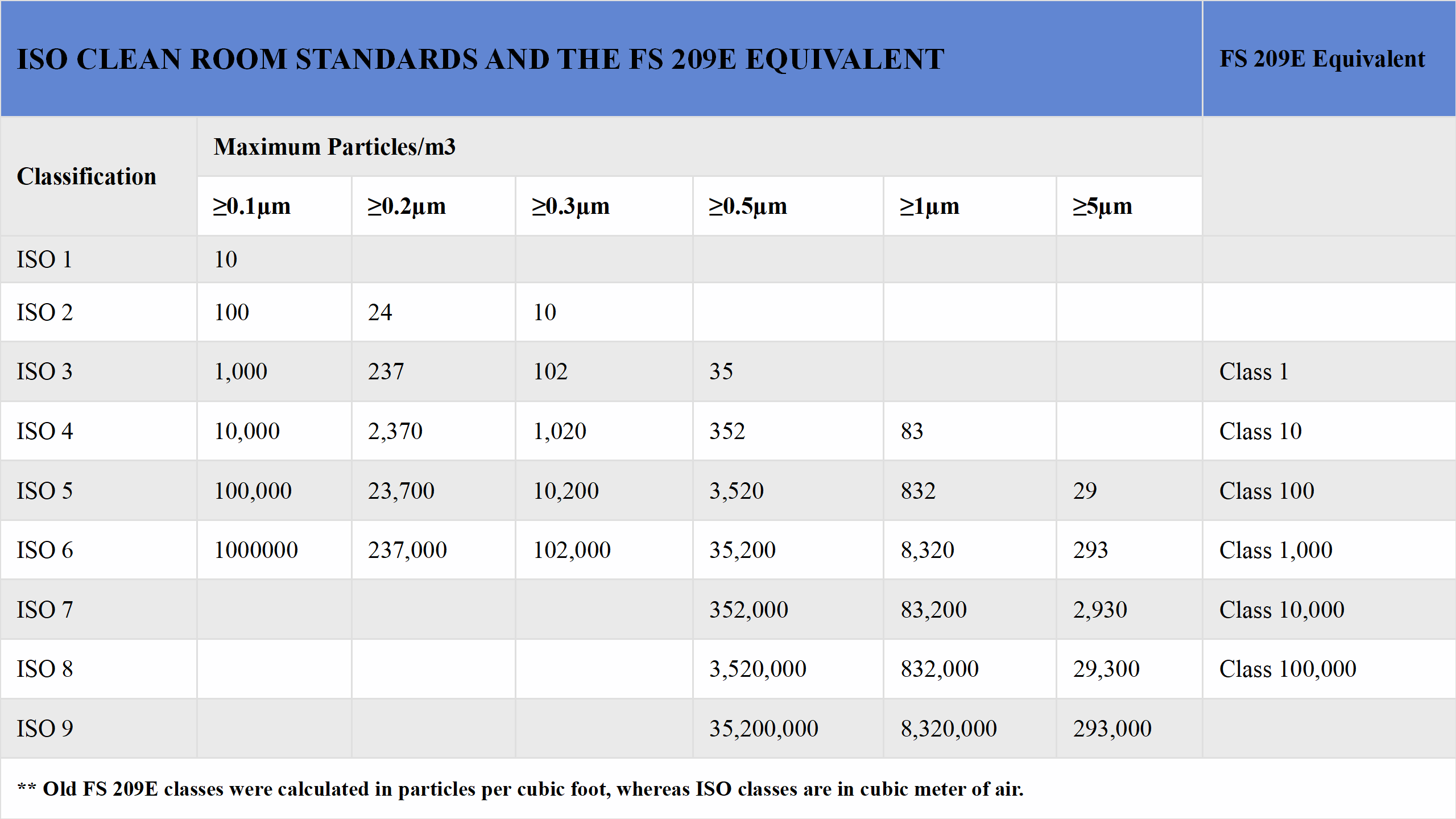
Particle limits are a cornerstone of cleanroom design. For ISO 8 cleanrooms, the standard specifies that the number of particles 0.5 microns or larger must not exceed 3,520,000 per cubic meter of air. To meet this threshold, the air is continuously filtered to control dust and microbial levels. This ensures a clean production environment, protecting products from contamination and maintaining their quality and reliability.
The ISO 8 classification focuses on maintaining air cleanliness by limiting particles 0.5 microns or larger to 3,520,000 per cubic meter. This level suits applications with moderate cleanliness needs, such as certain laboratories or less demanding electronics manufacturing processes. By meeting these standards, businesses can ensure their production spaces align with cleanliness requirements, enhancing product quality and customer trust.
ISO 8 cleanrooms rely on advanced air filtration systems to keep particle and microbial counts within specified limits. High-efficiency filters continuously clean incoming air to meet ISO 8 requirements, ensuring a consistently clean environment.
Temperature, humidity, and pressure controls are also essential. Stable conditions reduce airborne particles, while positive pressure prevents external contaminants from entering. These measures create a reliable workspace for sensitive processes.
Controlled entry points further minimize contamination risks. Staff must pass through designated areas and follow strict protocols, such as hand washing, changing into cleanroom attire, and using air showers. These steps reduce the chance of introducing particles or microbes.
Additionally, personnel must adhere to strict dressing procedures, wearing sterile gowns, caps, gloves, and other personal protective equipment (PPE). These measures ensure that workers do not bring contaminants into the cleanroom, maintaining its cleanliness throughout operations.
ISO 8 cleanrooms are used in industries like pharmaceuticals, medical device manufacturing, and semiconductor production, where controlled environments are critical for product quality and safety.
In pharmaceuticals, ISO 8 cleanrooms are often used for producing sensitive ingredients or packaging finished products. For example, during filling, sealing, or packaging, a clean environment prevents contamination by microbes or particles. This pharmaceutical clean room ensures the safety and quality of medications.
In terms of clean room for medical devices manufacturing, ISO 8 cleanrooms support the assembly of precision components, such as surgical instruments or electronic parts. These processes require a dust-free environment to ensure product safety and performance. ISO 8 cleanrooms are also used for testing and assembling sterile devices, meeting strict hygiene standards.
In semiconductor production, ISO 8 cleanrooms are employed for tasks like wafer growth, dicing, cleaning, and assembly. These processes are highly sensitive to particles and static electricity, requiring a clean environment to ensure product reliability and performance.
● Protecting Products from Contamination. In industries requiring precision and cleanliness, even minor contaminants can compromise product quality. ISO 8 cleanrooms use strict controls to keep dust, microbes, and other particles out of the production process. In pharmaceuticals, for instance, contamination could pose serious health risks. ISO 8 cleanrooms help ensure drugs remain pure and safe throughout production.
● Ensuring Compliance with Regulatory Standards. Many industries face strict regulatory requirements, and ISO 8 cleanrooms help companies meet these standards. In pharmaceuticals and medical device manufacturing, compliance with hygiene and quality regulations is critical. ISO 8 cleanrooms simplify certification processes and provide assurance in case of quality audits. In semiconductor production, they support particle-free environments for high-precision tasks, ensuring reliable products.
● Achieving Cost-Effectiveness. While setting up and maintaining an ISO 8 cleanroom involves costs, the investment pays off over time. Higher product quality reduces rework and recalls, saving time and resources. A clean, efficient production environment also minimizes downtime and boosts equipment use, leading to long-term cost savings. Additionally, meeting industry standards can enhance a company’s reputation and help avoid penalties for non-compliance.
Compared to stricter classifications like ISO 7 or ISO 6, ISO 8 has more lenient particle limits. ISO 7 Clean Room allows no more than 3,520 particles of 0.5 microns per cubic meter, while ISO 6 permits only 120 particles of 0.1 microns per cubic meter. ISO 8, with its higher particle allowance, is more cost-effective for applications with moderate cleanliness needs.
Choosing the right cleanroom class depends on the application. For precision medical devices or cosmetics, ISO 7 or ISO 6 Cleanroom may be necessary to meet stringent cleanliness requirements. For less demanding tasks, such as non-sterile production or general lab work, ISO 8 cleanrooms provide sufficient protection against contaminants while remaining affordable.
Maintaining an ISO 8 cleanroom requires ongoing monitoring, maintenance, and certification to ensure consistent cleanliness. Key challenges include contamination control, air flow management, and equipment standards.
Contamination control is critical, as even small amounts of dust or microbes can affect product quality. Regular cleaning, disinfection, and strict entry protocols are necessary to keep the cleanroom free of external contaminants.
Air flow management is equally important. The air must be continuously filtered to meet ISO 8 particle limits. Poor air flow can lead to particle buildup, compromising product quality. Regular maintenance of air handling systems ensures proper performance.
Equipment used in ISO 8 cleanrooms must also meet strict standards to avoid introducing contaminants. Production and testing equipment should be regularly inspected and calibrated to maintain optimal condition and support a clean production process.
What Is an ISO 5 Cleanroom Classification?

Wiskind Cleanroom specializes in cleanroom enclosure system , ceiling system, cleanroom doors and windows and related product development, manufacturing, sales, consulting and services.