We have a passion for unconventional solutions that bring your vision to life.
Designing a regulatory-compliant cleanroom—whether for GMP, GTP, clinical manufacturing, or pharmacy compounding—requires specialized expertise, careful planning, and alignment with strict industry standards. Our team of engineers, architects, drug manufacturing specialists, and certified cleanroom professionals has unmatched experience in every phase of cleanroom design, commissioning, and operations, linking facility design to the clinical and regulatory needs that drive project success. From early planning to long-term operations, we help life sciences organizations meet tough product quality, safety, and compliance requirements through smart cleanroom design. Below, we combine our full-spectrum cleanroom solutions with a step-by-step guide to designing effective, regulatory-ready cleanrooms—simplifying the process while highlighting key technical and compliance factors.
Wiskind Cleanroom Expertise & Integrated Solutions
We offer end-to-end support for cleanrooms across the life sciences industry, with solutions tailored to meet regulatory standards and evolving operational needs. Our integrated team supports every phase of cleanroom design, commissioning, and operations, delivering results that align with both technical performance and regulatory requirements. Whether you need to improve an existing cleanroom or build one from scratch, our expertise ensures your cleanroom design meets all performance and compliance goals.
We design and build cleanrooms that comply with 21 CFR Parts 210, 211, 212, and 1271, including HCT/P requirements and quality systems for clinical trials and commercial manufacturing—key components of a compliant cleanroom design. Our approach ensures every aspect of your design adheres to these strict regulations.
From Phase 1 to Phase 3 clinical trials, we help clients design and expand cleanrooms to support growing production needs while maintaining compliance. Our flexible cleanroom design solutions let you scale without sacrificing quality.
Wiskind experts support medical device and pharmaceutical clients in bringing new drug and biologic delivery systems to market through risk assessment, engineering design, program planning, and regulatory guidance.
We help pharmacies and healthcare facilities meet compounding standards by reviewing engineering and administrative controls, workflows, and environmental practices, and providing gap assessments and action plans.
Wiskind team creates and updates SOPs for compounding operations to meet USP, FDA, and state requirements. We also provide staff training on aseptic techniques, gowning, HVAC systems, environmental monitoring, and more.
Wiskind design and commission cord blood banking facilities with a focus on FDA-compliant workflows, construction quality control, critical systems redundancy, and environmental monitoring protocols.
Wiskind experts assess and mitigate HD exposure risks across healthcare settings. Ensure sustainable USP <800> compliance for pharmacy, nursing, and facility operations with guidance from our occupational health and toxicology experts.
We provide full services from planning and design to modular/mobile spaces, commissioning, and as-built documentation—ensuring your cleanroom operates effectively, scales easily, and remains flexible throughout every stage of design and construction. Our end-to-end approach eliminates gaps in your design for a more efficient facility.
Our team performs equipment IQ/OQ, ISO cleanroom certification, and continuous quality control. We help guide ongoing operations through environmental monitoring committee membership and regulatory audits.
While "easy" may not be the first word that comes to mind when designing sensitive cleanrooms, a logical, step-by-step approach creates a solid, compliant cleanroom design. Below is a detailed breakdown of each key step, including practical tips for adjusting load calculations, planning exfiltration paths, and allocating mechanical room space—all aligned with our expertise in regulatory compliance and efficiency. This framework covers critical factors like people/material flow, cleanliness classification, and mechanical system design, helping you avoid common mistakes and build a cleanroom that meets both performance and regulatory needs.
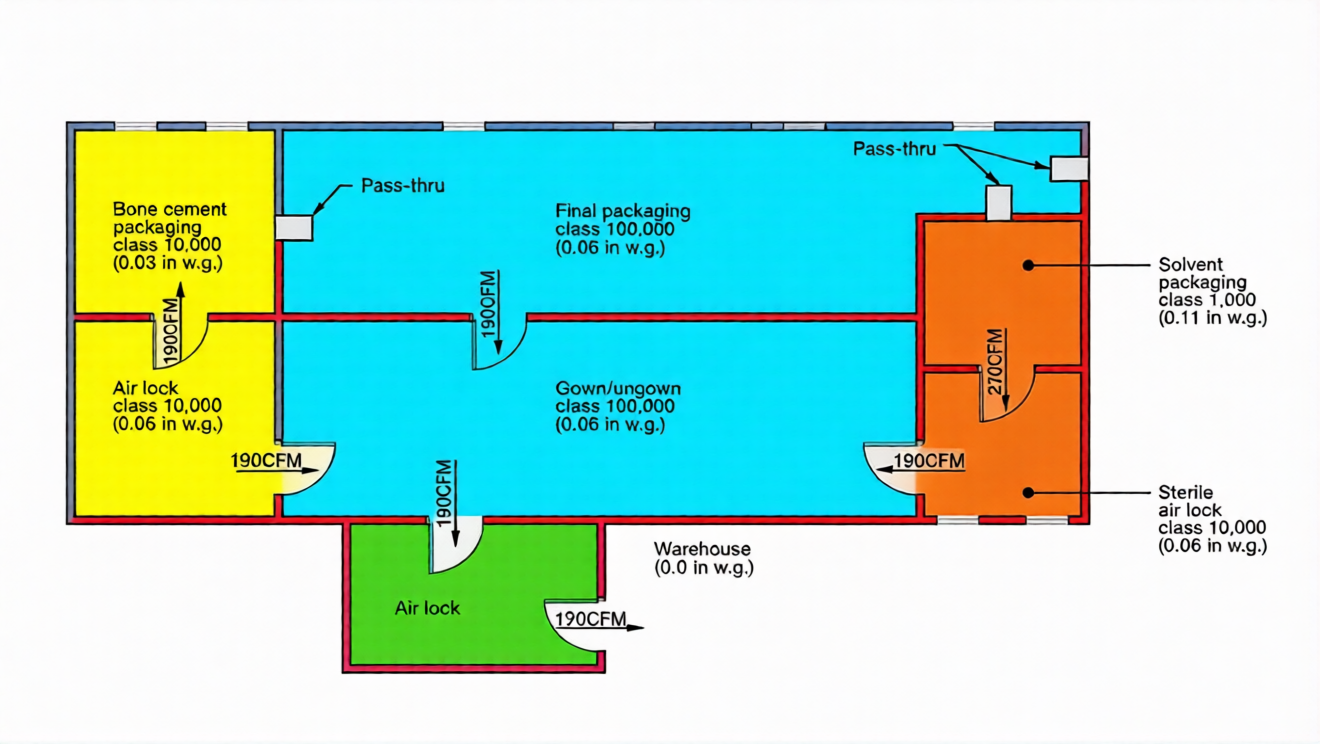
Cleanroom workers are the biggest source of contamination, so keeping critical processes away from personnel doors and walkways is key to a good cleanroom design. Critical spaces should have one access point to avoid becoming a pathway to less critical areas—an important layout consideration. You must also carefully check for process cross-contamination, including raw material inflow, material isolation, and finished product outflow. For example, in a bone cement facility, critical spaces like "Solvent Packaging" and "Bone Cement Packaging" have single access points, with airlocks (e.g., "Gown" and "Ungown" areas) acting as buffers for high-traffic zones, reducing contamination risk and improving overall design.
The main guide for cleanliness classifications is IEST Standard 14644-1, which defines classes (1, 10, 100, 1,000, 10,000, 100,000) based on allowed particle counts at different sizes—a key part of cleanroom design. For example, a Class 100 cleanroom allows up to 3,500 particles/cu ft (0.1 microns and larger), 100 particles/cu ft (0.5 microns and larger), and 24 particles/cu ft (1.0 microns and larger). Choosing a class depends on process sensitivity, reject rates, and regulatory guidelines (like FDA rules) that shape your design. Picking the right class balances performance and cost.
A key rule for cleanroom design: connecting spaces should not have more than two orders of magnitude difference in classification. For example, a Class 100,000 (ISO 8) cleanroom can open into a Class 1,000 (ISO 6) cleanroom but not a Class 100 (ISO 5) cleanroom—this prevents contamination and ensures compliance. In our bone cement facility example: "Gown/Ungown" and "Final Packaging" are Class 100,000 (ISO 8); "Bone Cement Airlock" and "Sterile Airlock" are Class 10,000 (ISO 7); "Bone Cement Packaging" (a dusty critical process) is Class 10,000 (ISO 7); and "Solvent Packaging" (a very critical process) uses Class 100 (ISO 5) laminar flow hoods within a Class 1,000 (ISO 6) cleanroom—all following best design practices.
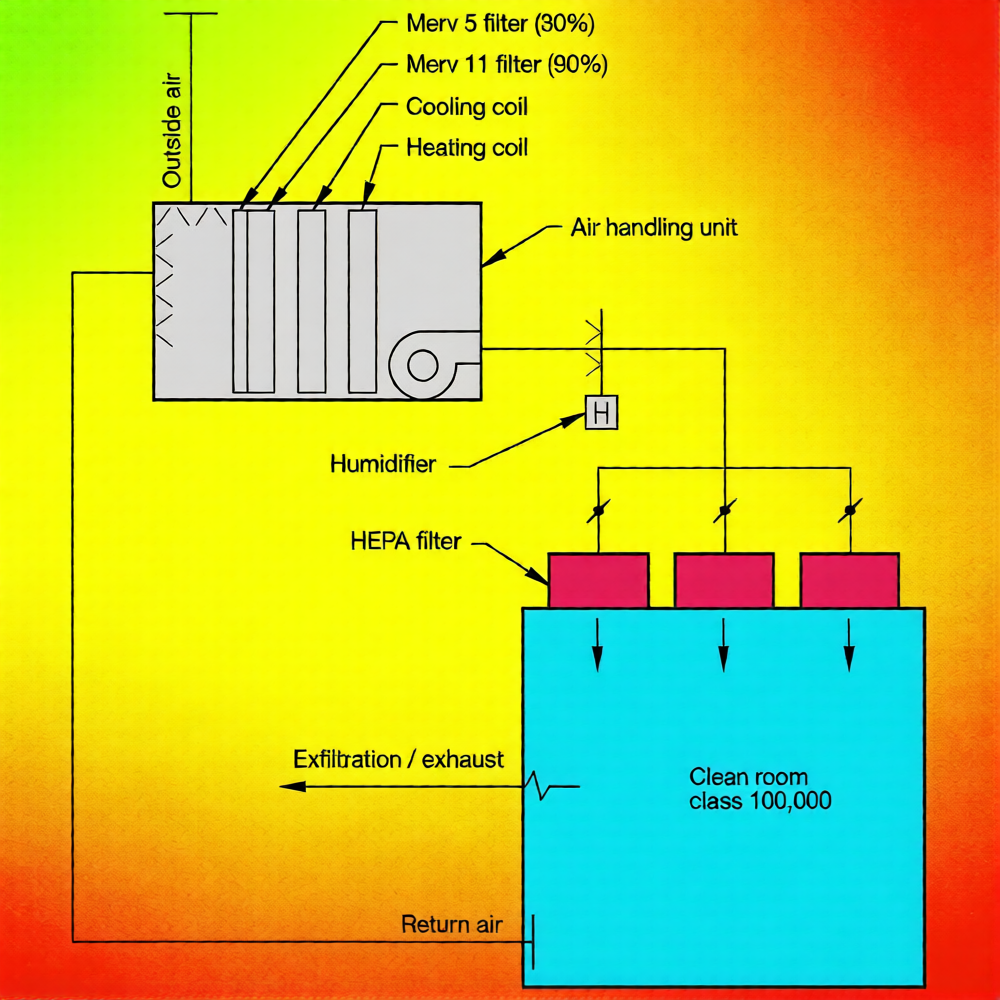
Maintaining positive air pressure relative to dirtier adjacent spaces is critical to preventing contamination—neutral or negative pressure makes it nearly impossible to maintain consistent classification. That’s why pressure control is key to cleanroom design. Studies show a pressure difference of 0.03 to 0.05 in w.g. effectively reduces contamination; higher differences (above 0.05 in w.g.) offer little extra benefit but increase energy costs and the force needed to operate doors—important tradeoffs to consider. The recommended maximum pressure difference across a door is 0.1 in w.g. (a 3ft x 7ft door requires 11 pounds of force to open or close), a detail that keeps your design practical.
In our bone cement facility (housed in an existing warehouse with neutral pressure, 0.0 in w.g.): "Gown/Ungown" has 0.03 in w.g.; "Bone Cement Air Lock," "Sterile Air Lock," and "Final Packaging" have 0.06 in w.g.; "Bone Cement Packaging" has 0.03 in w.g. (lower to contain dust); and "Solvent Packaging" has 0.11 in w.g. (no structural reinforcement needed—pressures above 0.5 in w.g. require structural checks). This pressure setup is tailored to the facility’s needs and reflects thoughtful cleanroom design that balances contamination control and practicality.
Cleanliness classification is the main factor in setting supply airflow, measured by air changes per hour (ach)—a critical consideration for cleanroom design. IEST Standard 14644-4 provides recommended ach ranges: for example, Class 100,000 (ISO 8) uses 15–30 ach. Adjustments depend on room activity: a low-occupancy, low-particle cleanroom may use 15 ach, while a high-occupancy, high-traffic space may need 30 ach—customizations that keep your design efficient. Other factors include process exhaust and air flow through doors/openings, all of which must be included in your design.
In our bone cement facility: "Gown/Ungown" (high traffic, non-critical) uses 20 ach; "Sterile Air Lock" and "Bone Cement Air Lock" (critical buffers) use 40 ach; "Final Packaging" (non-critical secondary packaging) uses 20 ach; "Bone Cement Packaging" (critical) uses 40 ach; and "Solvent Packaging" (very critical, ISO 6 cleanroom with ISO 5 hoods) uses 150 ach. Clean air comes from HEPA filters—more air changes mean fewer airborne particles, a key principle for meeting classification standards in cleanroom design.
Most cleanrooms have positive pressure, leading to planned air flow to adjacent lower-pressure spaces and unplanned air leakage through electrical outlets, light fixtures, and door/wall/floor connections—a factor you can’t ignore in cleanroom design. A well-sealed cleanroom has a 1–2% volume leakage rate—zero leakage is impossible, a fact that guides practical design. For active supply/return/exhaust control, you need a minimum 10% difference between supply and return airflow to keep air valves working independently, a technical detail that ensures design stability. Air leakage through doors depends on door size, pressure difference, and sealing (gaskets, door drops)—all part of a complete cleanroom design.
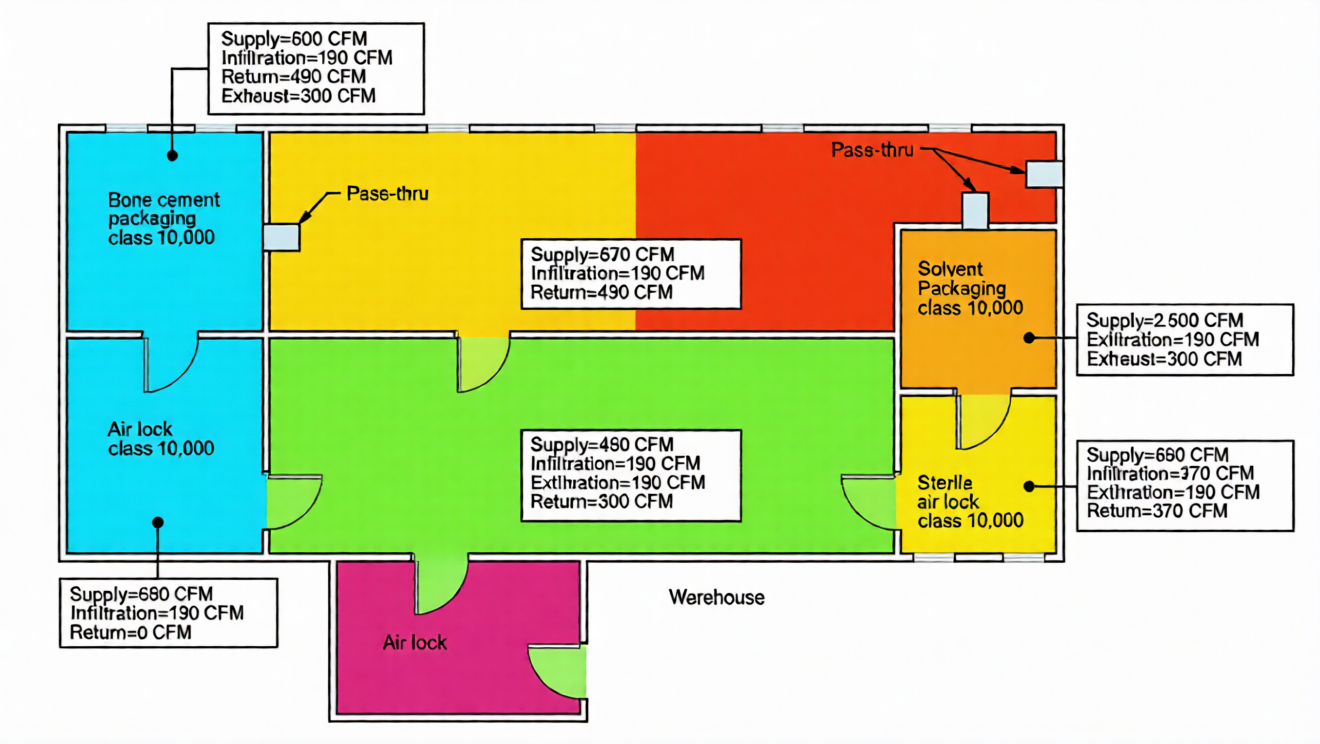
In our bone cement facility, a 3ft x 7ft door has 190 cfm leakage at 0.03 in w.g. difference and 270 cfm at 0.05 in w.g. Unplanned leakage usually escapes through wall stud spaces and the top of the wall—understanding this flow helps optimize sealing and airflow in your cleanroom design, preventing unnecessary contamination and energy waste.
Air balance means total air entering a space (supply + infiltration) equals total air leaving (exhaust + exfiltration + return)—a basic principle of cleanroom design that ensures consistent performance. For example, in our bone cement facility: "Solvent Packaging" has 2,250 cfm supply, 270 cfm leakage to "Sterile Air Lock," and 1,980 cfm return; "Sterile Air Lock" has 290 cfm supply, 270 cfm air from "Solvent Packaging," 190 cfm leakage to "Gown/Ungown," and 370 cfm return. Final return airflow is adjusted during start-up to account for unplanned leakage, a step that fine-tunes your design for optimal performance.
Beyond airflow and pressure, several factors affect cleanroom performance and compliance—all critical to refining your design. Overlooking these details can reduce your cleanroom’s effectiveness, leading to non-compliance or inefficiency. Below are the key factors to address:
Temperature: Maintain 66°F–70°F for worker comfort (due to smocks/bunny suits that reduce heat dissipation).
Humidity: The optimal relative humidity (RH) is 45% +/-5% to reduce electrostatic charge. High charge attracts particles, which can be released suddenly; low RH also damages ESD-sensitive materials.
Laminarity: Very critical processes may require laminar flow (per IEST Standard IEST-WG-CC006) to prevent contaminants between HEPA filters and the process.
Electrostatic Discharge (ESD): ESD-sensitive processes may require grounded conductive flooring.
Noise & Vibration: Precision processes may need noise/vibration control to maintain accuracy.
Mechanical system design depends on space, budget, process needs, cleanliness classification, reliability, energy costs, building codes, and climate—all key to successful cleanroom design. Unlike standard A/C systems, cleanroom systems require far more supply air than just for heating and cooling, a key difference in mechanical planning. Choosing the right mechanical layout ensures your design is efficient, reliable, and compliant.
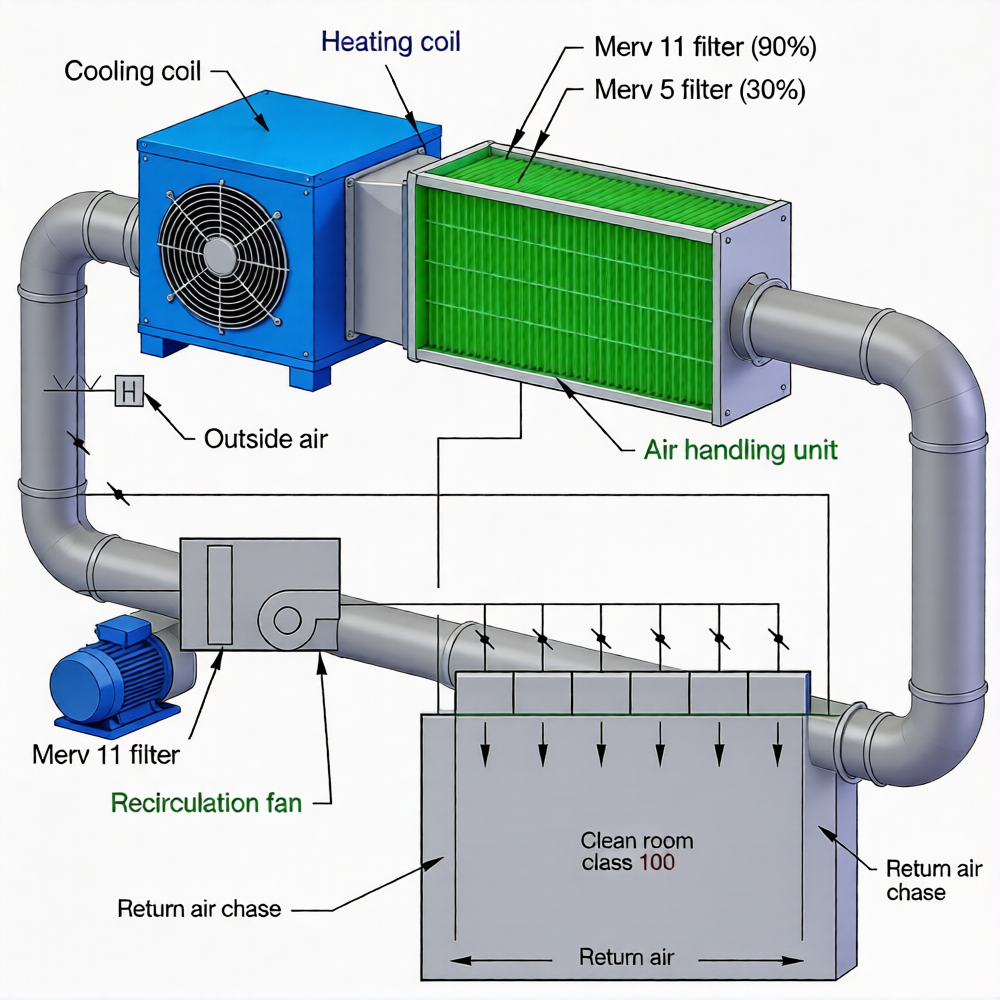
Class 100,000 (ISO 8) and low-ach Class 10,000 (ISO 7) cleanrooms can use a single air handling unit (AHU): return and outside air are mixed, filtered, cooled, reheated, humidified, and sent to ceiling HEPA filters, with low wall returns to prevent recirculation—a cost-effective mechanical solution for basic designs. Higher-ach Class 10,000 (ISO 7) and cleaner cleanrooms use a split system: part of the return air goes to the AHU for conditioning, while the rest returns to a circulation fan—a more robust setup for advanced cleanroom design.
Fan Filter Units (FFUs)—modular filtration solutions—are an alternative to traditional AHUs, suitable for ISO 3 to ISO 8 cleanrooms. Ceiling coverage varies by class: 5–15% for ISO 8, 60–100% for ISO 3 or cleaner. FFUs add flexibility to your cleanroom design, making them ideal for facilities that may need to scale or adapt later.
Accurate heating/cooling load calculations are essential for cleanroom design, as they ensure worker comfort, energy efficiency, and consistent environmental conditions. Overlooking these calculations can lead to costly waste or non-compliance. To get accurate results, consider these factors:
• Conservative climate conditions (99.6% heating design, 0.4% drybulb/median wetbulb cooling design, and 0.4% wetbulb/median drybulb cooling design).
• Filtration heat gains.
• Humidifier manifold heat.
• Process loads (equipment, materials).
• Recirculation fan heat.
Cleanrooms require significant mechanical and electrical support, and the cleaner the class, the more support space you need—this is a key consideration that affects long-term functionality. For a 1,000-sq-ft cleanroom: Class 100,000 (ISO 8) needs 250–400 sq ft of support space; Class 10,000 (ISO 7) needs 250–750 sq ft; Class 1,000 (ISO 6) needs 500–1,000 sq ft; Class 100 (ISO 5) needs 750–1,500 sq ft. Allocating enough support space early in your cleanroom design prevents bottlenecks and makes it easier to maintain and upgrade the facility.
Actual support space depends on AHU complexity (simple: filter, coils, fan; complex: sound attenuator, return fan, relief air, humidifier, etc.) and the number of dedicated systems (exhaust, recirculation, chilled water, steam, DI/RO water). Share your support space needs with your project architect early—this proactive step ensures your cleanroom design includes enough space, avoiding costly changes later.
Cleanrooms are high-performance systems—when well-designed and built, they operate efficiently and reliably, meeting regulatory and operational needs. When poorly designed, they are costly to maintain and prone to compliance failures—highlighting why quality cleanroom design matters. Our team’s expertise in GMP/GTP compliance, clinical manufacturing, and cleanroom engineering complements this step-by-step guide, helping you navigate challenges and avoid common mistakes. For your first few cleanroom projects, working with an engineer with extensive experience (like our certified professionals) is highly recommended to ensure regulatory readiness and long-term performance. Remember, every detail of your cleanroom design contributes to its success, from layout and classification to mechanical systems and support space.

Wiskind Cleanroom specializes in cleanroom enclosure system , ceiling system, cleanroom doors and windows and related product development, manufacturing, sales, consulting and services.