We have a passion for unconventional solutions that bring your vision to life.
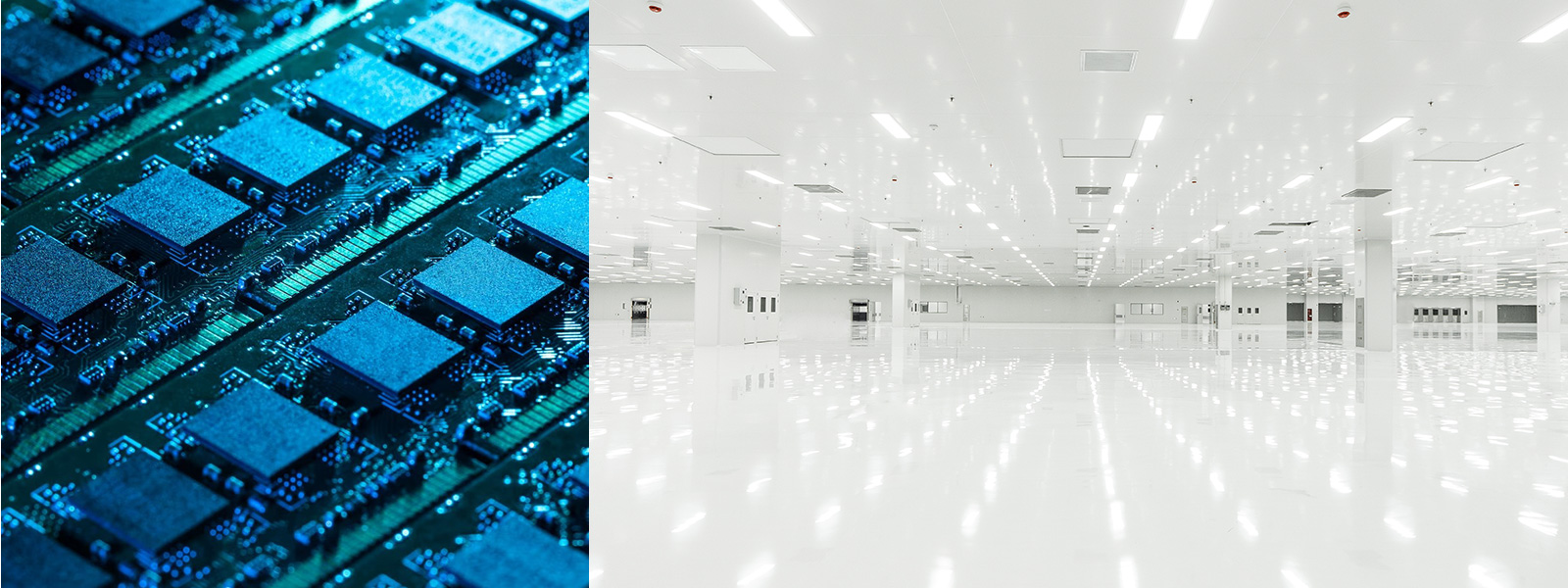
Semiconductor Cleanroom
According to the standards of the International Semiconductor Equipment and Materials Association (SEMI) and related domestic standards, the control of particulate matter and molecular contaminants is stringent. For instance, advanced processes at 10 nanometers or below often require ISO Class 1 or higher, meaning no more than 10 particles greater than or equal to 0.1 micrometers per cubic meter of air.
Pharmaceutical Cleanroom
Complies with Good Manufacturing Practice (GMP) standards. Different levels have various limits on microorganisms and particles. High-risk operating areas for sterile drug production are typically Class A, equivalent to ISO Class 5, with no more than 3520 particles greater than or equal to 0.5 micrometers per cubic meter, and no more than 1 CFU/m³ of airborne bacteria.
Semiconductor Cleanroom
Focuses on controlling particulate contaminants, molecular contaminants, and static electricity. Tiny particles can affect photolithography and other processes. Molecular contaminants like organic volatiles and metal ions can pollute the chip surface, and static electricity can lead to chip breakdown.
Pharmaceutical Cleanroom
Mainly controls microbial contamination, temperature, humidity, and pressure differentials. Microorganisms can contaminate drugs, affecting their quality and safety. Appropriate temperature and humidity facilitate drug production and personnel operation, while reasonable pressure differentials prevent cross-contamination between different areas.
Commonly adopts a "chip manufacturing area-auxiliary equipment area-clean support area" layout. The chip manufacturing area includes core processes like photo lithography and etching, arranged linearly or in a ring according to the process flow to shorten material transfer distances and reduce particle generation and introduction.
Pharmaceutical Cleanroom
Laid out according to the drug production process and cleanliness level requirements, including areas for ingredient preparation, granulation, table-ting, and packaging. Sterile drug production also includes aseptic operation areas and cleaning and disinfection areas, emphasizing the separation of personnel and material flows to prevent cross-contamination.

Semiconductor Cleanroom
Equipment often includes high-precision photo lithography machines, etching machines, etc., which are sensitive to vibrations, temperature, and humidity. High-precision temperature and humidity control systems and ultra pure water systems are needed. Construction materials need to be low-dust, anti-static, and corrosion-resistant, such as anti-static flooring and color steel plates.
Equipment includes pharmaceutical production lines and sterilization equipment, emphasizing equipment cleanliness and disinfection. Materials in contact with drugs should be corrosion-resistant and easy to clean, such as stainless steel. Construction materials should meet hygiene requirements, with smooth, seamless walls and floors for easy cleaning and disinfection, commonly using epoxy self-leveling floors and antibacterial coating walls.
Semiconductor Cleanroom
Staff must wear anti-static, dust-free clothing and enter through strict purification procedures like air showers. Operations should be performed gently to minimize particle generation and dispersal, requiring high skills and proficiency from personnel.
Pharmaceutical Cleanroom
Personnel must wear clean work clothes and undergo strict hygiene procedures, such as handwashing, disinfection, and changing into clean garments. Regular microbial testing and health checks are conducted to prevent personnel from bringing microorganisms into the cleanroom.

Wiskind Cleanroom specializes in cleanroom enclosure system , ceiling system, cleanroom doors and windows and related product development, manufacturing, sales, consulting and services.